ophthafutur®
octa | deca
Ultra-purified perfluorocarbon liquids largely used in vitreoretinal surgery as intraoperative tools.
The purity of these liquids is of crucial importance for the safety of the treated eye: to guarantee the best possible surgical outcomes all harmful contaminants, e.g. reactive and under-fluorinated compounds are removed from our products.
- High patient and product safety
- Validated and controlled multi-step ultra-purification process
- Safe, biocompatible, sterile, endotoxin-free products
- Novel high-performance polymer syringe (HPPS)
- 36 months shelf life

What are perfluorocarbon liquids?
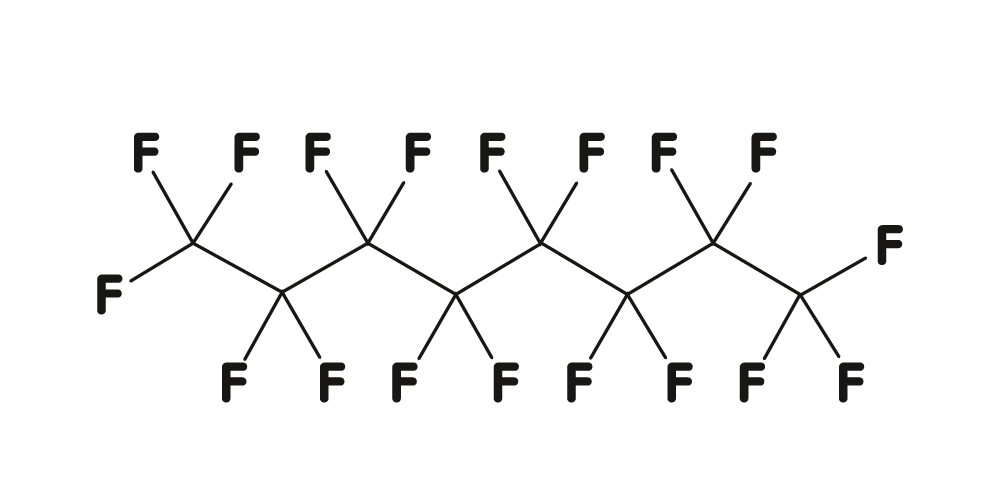
Perfluorocarbon liquids (PFCL) belong to a family of synthetic compounds which are derived from hydrocarbons and in which the hydrogen atoms are completely replaced by fluorine atoms. Due to their structure they are inert, colorless and odorless.
In the last decades, PFCL have been used as strong tools in ophthalmology, artificial blood substitution and oxygen transport.
For all these medical applications a high degree of purity, only attainable through an intensive ultra-purification enabling a broad and accurate detection of impurities, is required.
The Challenge
Manufacturing-related impurities
Perfluorooctane and Perfluorodecaline belong to the family of PFCL. These fully fluorinated compounds have a high safety profile in medical applications due their inert character. However, it must be noted that they can contain different kinds of impurities derived from the industrial manufacturing process, and these can jeopardize their safety profile.
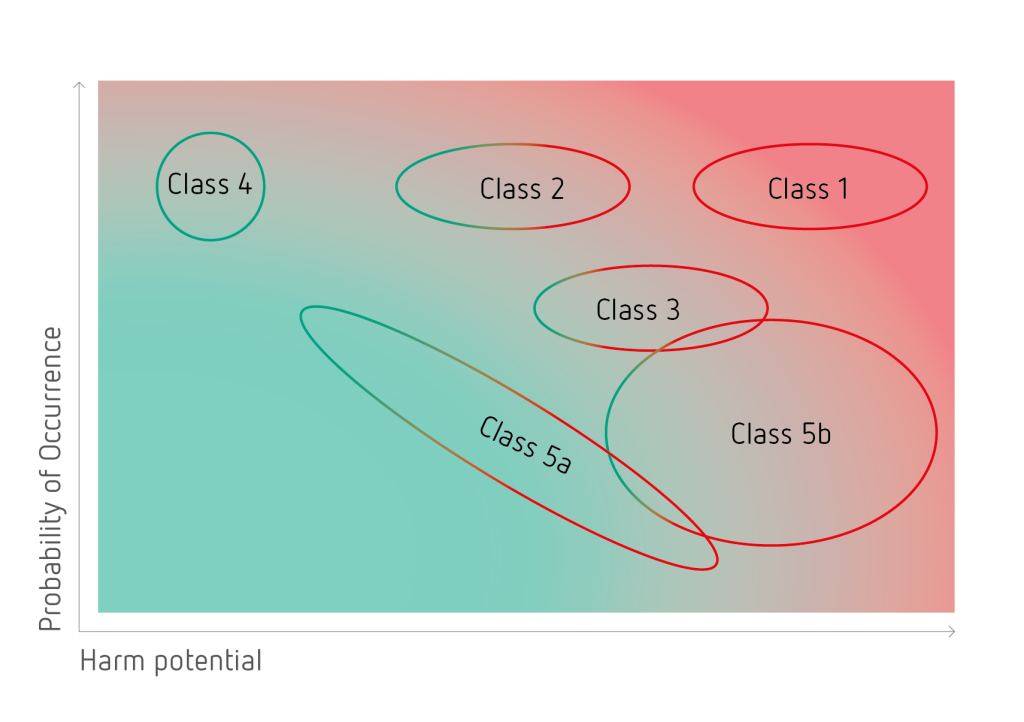
In order to obtain a safe product it is crucial to identify all possible impurities. To this purpose a matrix, classifying all different types of impurities based on their harm potential and probability of occurrence, has been created.
D.H. Menz et al. Hydrofluoric Acid and Other Impurities in Toxic Perfluorooctane Batches, Trans. Vis. Sci. Tech. (2019) 8(3):24.
In total 6 different impurity classes have been identified. Amongst these, class 1 impurities can be considered the most critical with a high harm potential in combination with a high probability of occurrence. Impurities belonging to class 1 have been identified to cause severe damages to the sensitive retinal tissues. Generally, all critical impurity classes have to be intensively controlled and completely removed.
Class 1
Reactive underfluorinated compounds
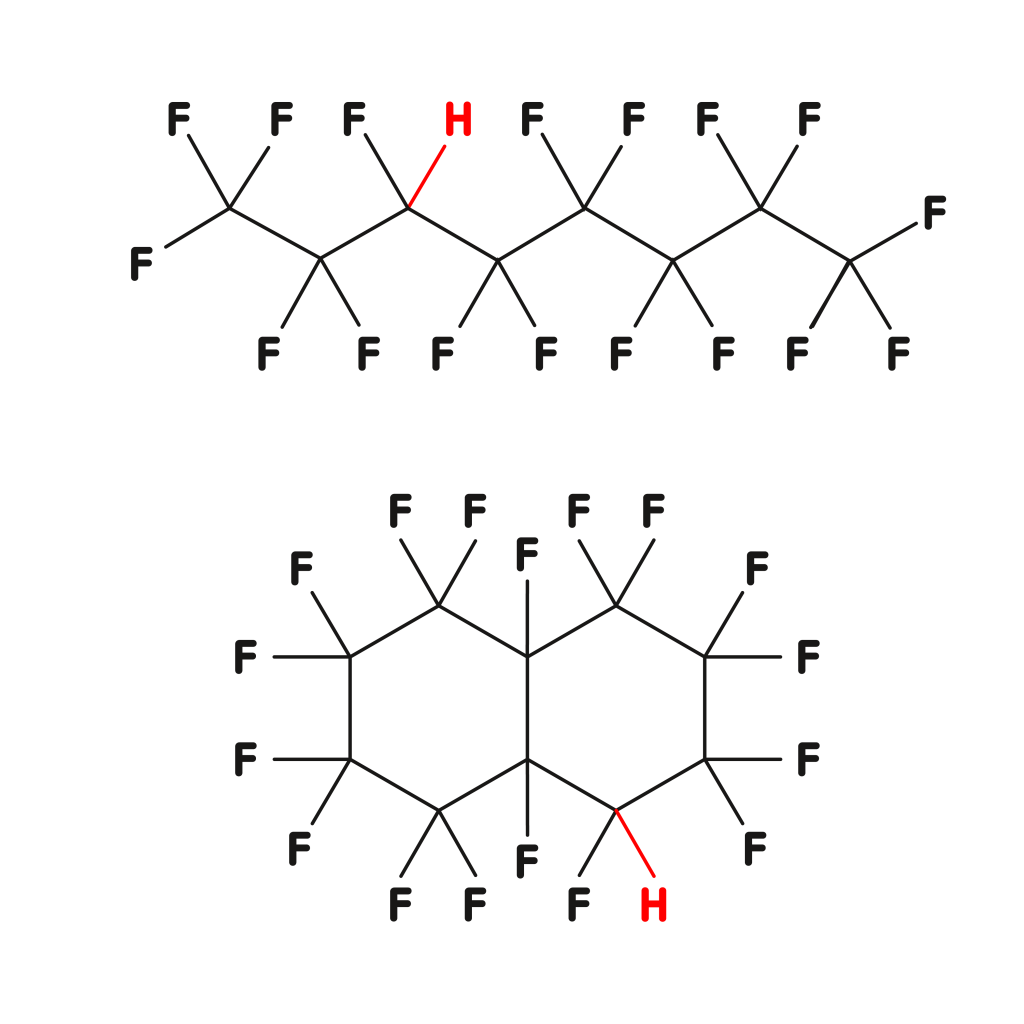
- unavoidable by-products of the PFCL synthesis
- very high harm potential and probability of occurrence
- the H-value is a suitable analytical tool
Class 2
Non-reactive underfluorinated compounds
- unavoidable by-products of the PFCL synthesis
- low to medium harm potential
- controlled by applying specifically optimized analytical methods and tools
Class 3
Surface-active contaminants
- compounds with amphiphilic character
- medium to high harm potential
- controlled by applying specifically optimized analytical methods and tools
Class 4
Fully fluorinated by-products
- by-products of the PFCL synthesis
- no harm potential
Class 5a
Non-PFCL-specific contaminants – UV leachables
- impurities derived from storage containers
- low to high harm potential
- controlled by applying specifically optimized analytical methods and tools
Class 5b
Non-PFCL-specific contaminants – manufacturing related compounds
- process chemicals and manufacturing related compounds
- medium to high harm potential
- controlled by applying specifically optimized analytical methods and tools
In addition to the in-depth knowledge of the impurity classes, a comprehensive understanding of their possible altering behavior needs to be taken into account. In fact, specifically underfluorinated compounds are potentially reactive during the product shelf life, which can lead to an increased harm potential of an individual impurity.
This explains how the safety profile of a product may change over time provoking different unwanted side effects. This can also explain the remarkable qualitative differences observed between brands, and even single batches, available on the market.
J.H Dresp: Benchmarking different brands of perfluorocarbon liquids, Graefes Arch Clin Exp Ophthalmol. (2021 Jan) 259(1):21-27.
The Solution
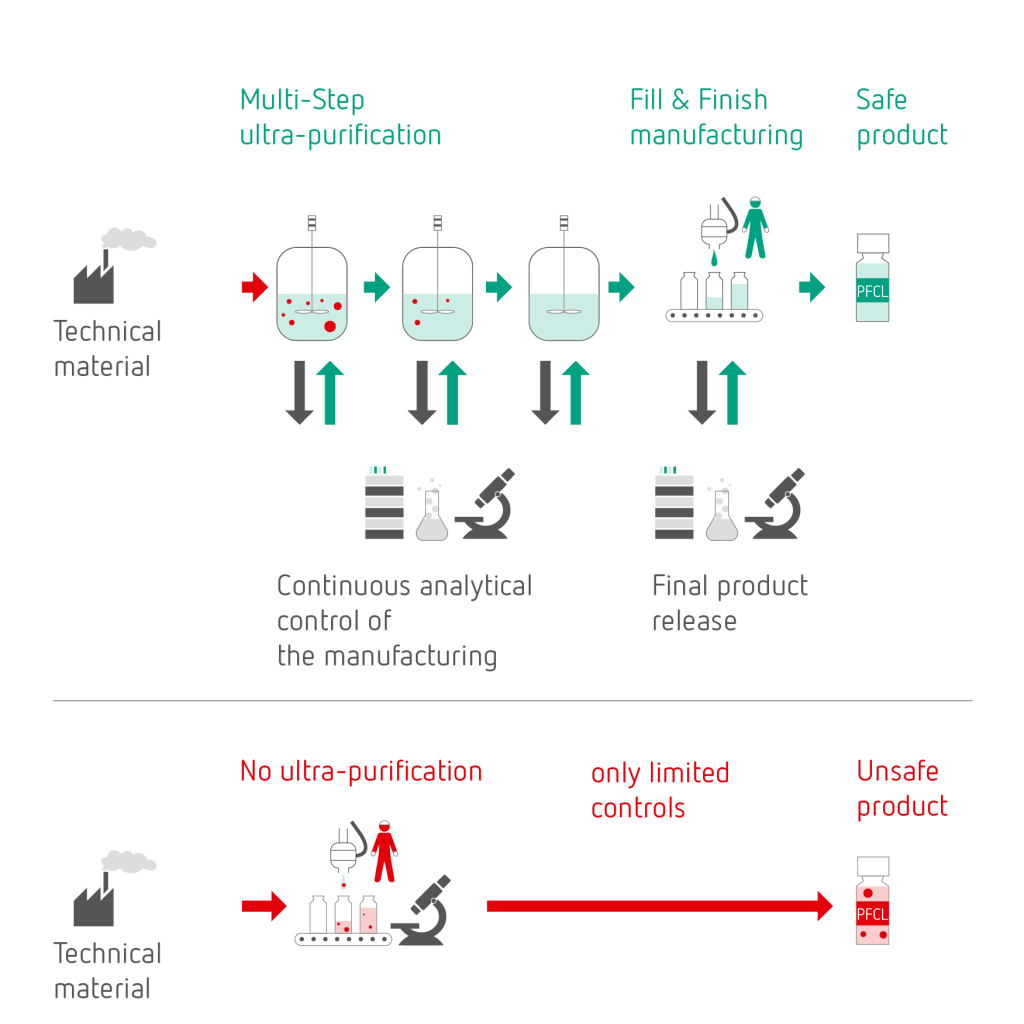
Multi-step ultra-purification process
To obtain a safe PFCL product the presented risk classification matrix needs to be considered and a suitable manufacturing process, including analytical measures, needs to be implemented. This enables the detection of all potential impurities, their removal and their control by appropriate tools and specification limits.
This is how ophthafutur® octa | deca are not simply purified: they are ultra-purified.
Using a validated and fully controlled unique multi-step manufacturing process, based on a profound technical know-how, a sound risk analysis and a complete set of optimized and validated analytical methods and tools, ultimately safe products are obtained.
Objective evidence of the deep knowledge we, the ophthafutur® manufacturer, own is provided by our active engagement in identifying the root cause of the reported toxic effects provoked by contaminated PFCL batches in Europe and Chile between 2013 and 2015.
We have been co-authors of several scientific publications and we have been directly co-operating with the Health Authorities contributing to the updating of the ISO 16672:2020 standards.
The H-value implementation, guaranteeing the removal of class 1 “reactive underfluorinated compounds”, is one of the outstanding outcomes of this collaboration.
Biological Safety
The biological safety of the above described ophthafutur® octa | deca perfluorocarbon liquids has been assessed in compliance with the ISO 16672:2020 as well as with the applicable ISO 10993-1 standard.
The products are:
• non cytotoxic
• non sensitizing
• non eye-irritating
• endotoxin-free
Usability
Perfluorocarbon liquids (PFCL) are used as surgical aid to unfold and reposition the retina in cases of retinal detachment / PVR / PDR, giant tears, ocular trauma; for laser coagulation and cryotherapy; for lifting of subluxated lenses and foreign bodies from the vitreous cavity.
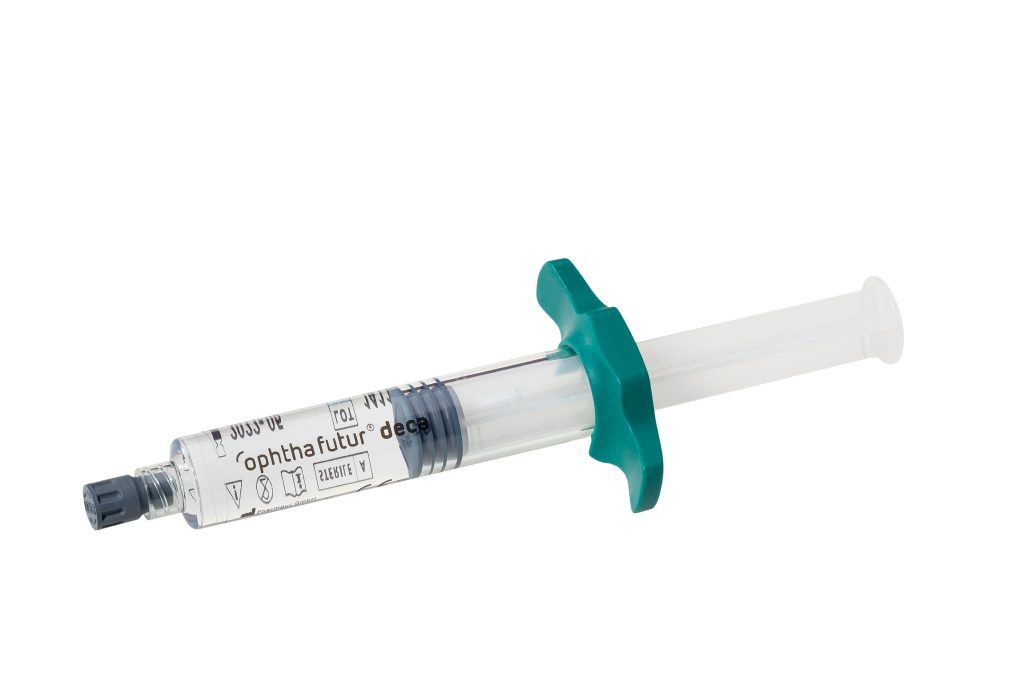
As PFCL filled glass syringes add considerable weight, which might be uncomfortable for some surgeons, and PFCL can possibly interact with the glass syringe lubricants, we introduced a novel material.
HPPS syringes avoid contact between the perfluorocarbon liquid and the standard lubrication used in glass syringes, thus increasing safety and reducing the likelihood of “sticky effects”.
Preloaded syringes offer an ideal solution for the surgeon.
For further in-depth information please contact your local distributor.